 The Centers for Disease Control and Prevention (CDC) with state and local health departments and the Food and Drug Administration (FDA) are investigating a multistate meningitis outbreak of fungal infections among patients who have received a steroid injection of a potentially contaminated product into the spinal area. Several patients suffered strokes that are believed to have resulted from their infection.
The Centers for Disease Control and Prevention (CDC) with state and local health departments and the Food and Drug Administration (FDA) are investigating a multistate meningitis outbreak of fungal infections among patients who have received a steroid injection of a potentially contaminated product into the spinal area. Several patients suffered strokes that are believed to have resulted from their infection.
The investigation also includes fungal infections associated with injections in a peripheral joint space, such as a knee, shoulder or ankle. CDC and public health officials are referring any patients who have symptoms that suggest possible meningitis or a possible peripheral joint infection to their physicians who can evaluate them further. Those patients injected in peripheral joints only are not believed to be at risk for fungal meningitis but could be at risk for joint infection.
Out of an abundance of caution, FDA released a statement advising physicians to follow-up with patients who received an injectable product, including an ophthalmic drug that is injectable or used in conjunction with eye surgery, as well as a cardioplegic solution purchased from or produced by the New England Compounding Center (NECC) after May 21, 2012. More >>
The Latest News on the Outbreak
- CDC continues to work with states to determine if there may be other fungal infections caused by exposure to NECC products beyond the three lots of preservative-free methylprednisolone acetate (80mg/ml) from NECC that were recalled on September 26, 2012. CDC does not have firm evidence that fungal infections have been caused by exposure to other NECC products.
- CDC’s guidance to patients has not changed as a result of the expanded voluntary recall of all New England Compounding Center (NECC) products, announced October 6. Patients who feel ill and are concerned about whether they received a medication from one of the NECC products recalled on September 26 should contact their physician.
- The type of meningitis that has been found in the investigation is caused by fungi (Exserohilum and Aspergillus) that are common in the environment but rarely cause meningitis. This form of fungal meningitis is not contagious.
- Patients and clinicians need to remain vigilant for onset of symptoms because fungal infections can be slow to develop. In this outbreak symptoms typically have appeared 1 to 4 weeks following injection, but it’s important to know that longer and shorter periods of time between injection and onset of symptoms have been reported. Therefore, patients and physicians need to closely watch for symptoms for at least several months following the injection. See updated Patient Guidance for more information, and contact your physician if you are concerned you may have become ill from your injection.
https://twitter.com/microbiology/status/258517145149915137
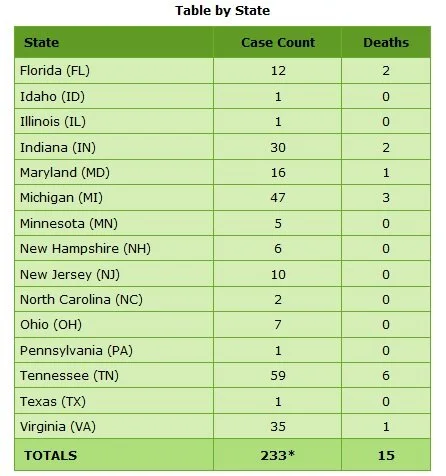
Map of the Affected US States
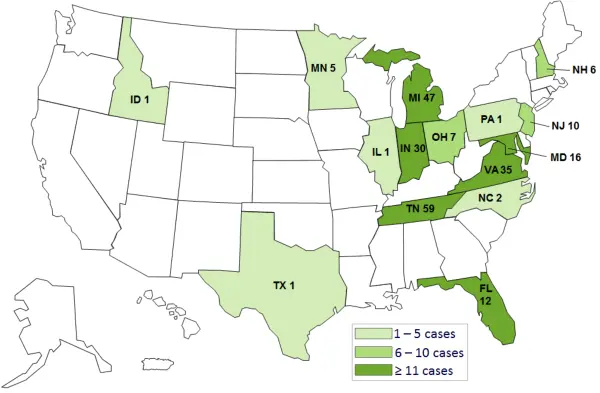
Click map for more information
Stay up to date with the latest news on the outbreak on Twitter:
- @Microbiology
- @CDCgov
